The team was launched in 2023. If you are motivated and would like to discuss the possibility of joining our projects either as a postdoc, PhD/Master student, or Technician/Assistant Engineer, please contact me at nicolas.panayotis@u-paris.fr.
Research themes
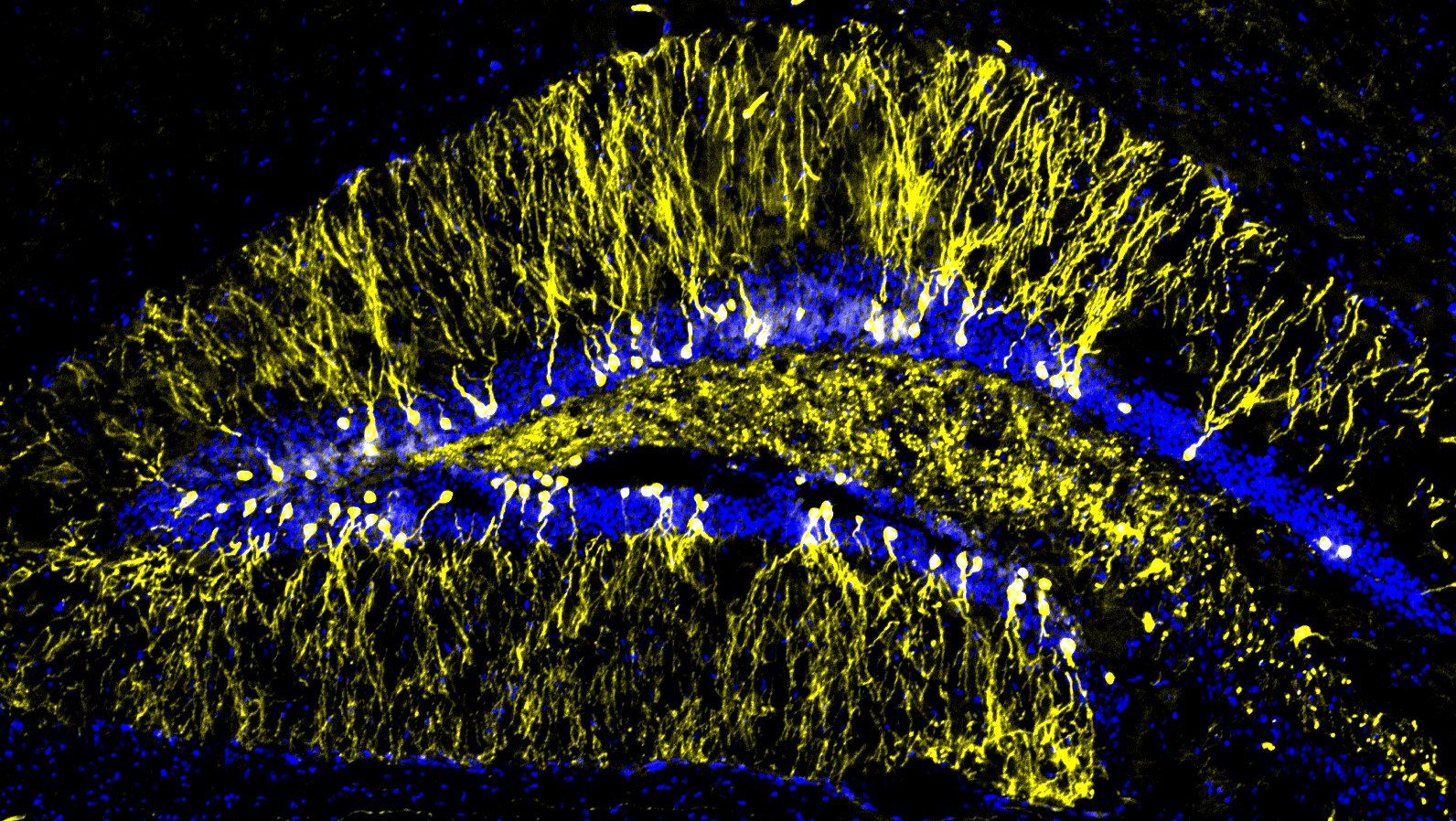
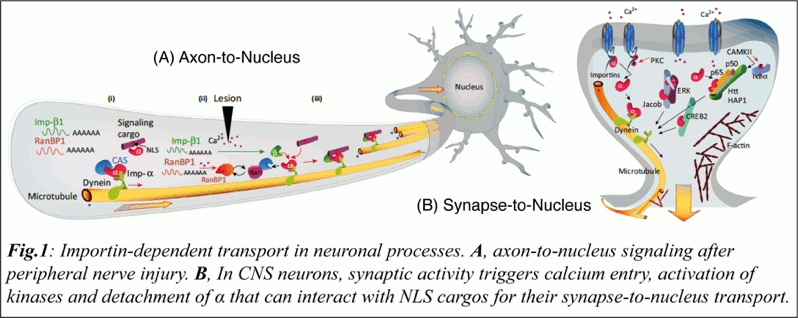
The importin family of nuclear import factors have pivotal roles in the subcellular transport of proteins from the cytoplasm and the synapse to the nucleus (Panayotis et al., 2015). While huge efforts helped to decipher the basic elements and properties of the nuclear transport, including the importin families of nuclear transport carriers (Schmidt and Gorlich, 2016), little attention has been paid to the roles of importins in the regulation of CNS functions.
In recent years, several studies, showed that importins have pivotal roles in the neuronal and nuclear transport of macromolecules during synaptic plasticity, maintenance, survival, and regeneration (Panayotis et al., 2015; Rishal and Fainzilber, 2014).
The mission of our team is to determine the roles of importins nuclear transport factors in neuronal functions and neurodevelopmental disorders, and to define the mechanistic rules of importin-mediated transport in physiologically-relevant networks. This approach will provide news findings and, importantly, news targets for therapeutic intervention in devastating neurodevelopmental disorders.
Lab projects
Click on titles to open.
1- Optimization of MeCP2 gene replacement therapy for Rett Syndrome
a. Boosting the brain delivery of viral vectors using focused ultrasound (fUS) technology.
Over the past years, several MeCP2 gene replacement strategies were tested in RTT mouse models with varying degrees of success (Jagadeeswaran et al., 2024).
Unfortunately, the number of transduced brain cells remained limited due to the low permeability of the blood-brain barrier (BBB) to AAVs. To overcome this limitations, intracerebral injections are often used, but this approach appears much harder to translate in clinics given the risks for the patients.
Focused ultrasound (fUS) coupled with circulating microbubbles (MB), is an innovative technology enabling temporary disruption of the BBB. MB are sensitizers of mechanical stress under bulk-pressures of varying amplitude. These are gas-filled and their size ranges from 1–5 μm. MB can repeatedly expand and compress when they are exposed to low acoustic pressures. This oscillation leads to tight junctions opening via mechanical stress on the endothelium. Many groups, validated this method to deliver AAVs in the brain and transduce a large number of brain cells in models of several pathologies such as RTT, ALS, AD, HD (Ye et al., 2024).
Goals: Increase brain delivery of the AAV-driven MeCP2 gene therapy vectors; maximize the transduction of disease-relevant brain cells
b. Manipulation of importin α-dependent transport in MeCP2 related disorders.
We identified two functionally relevant importin-transcription factors (TF) interacting pairs (α5-MeCP2 and α3-c-Fos). However, to date, the transport mechanisms controlling the abundance of TFs in the nucleus, and the transcriptional pathways at the core of neurodevelopmental disorders, have not been targeted in a systematic manner.
Thus, the main aim of our lab is to study the role of nuclear transport factors in neurogenetic brain disorders. Given the key role of MeCP2 in gene expression, both Rett syndrome (RTT) and MeCP2 fields tackled questions fundamental to the understanding of neuronal functions, gene expression, and brain disorders. MeCP2 mutations cause RTT, a devastating and progressive neurodevelopmental disorder while duplication of the MeCP2 locus cause severe cases of intellectual disabilities in MeCP2 Duplication Syndrome (MDS) patients.
Our priority in this aim will be to:
- Test how modulations of MeCP2 transport can rescue phenotypes associated with MeCP2-related disorders (Lombardi et al., 2015; Lyst and Bird, 2015). We will then expand the study to additional TFs and cellular/mouse models of genetic disorders.
- Characterize the basic rules of importin α-MeCP2’s interaction and transport dynamics using:
- Behavioral analyses,
- In vivo fUS imaging,
- live imaging of MeCP2 nuclear shuttling,
Goals: Hijack the importin-dependent transport of MeCP2 to titrate the level of the MeCP2 protein in the nucleus and avoid the potential overexpression phenotypes caused by untuned gene therapeutic strategies.
2 – Deciphering the roles of importins in CNS and PNS functions
Using a battery of approaches from behavior to molecular mechanisms, we discovered the role of importin α5 in anxiety pathways by regulating MeCP2 nuclear import in hippocampal neurons (Panayotis et al., 2018) and the role of importin α3 in the transport of c-Fos in dorsal root ganglia (DRG) neurons during chronic pain (Marvaldi et al., 2020). Our previous extensive behavioral screening of importin α single knockouts also revealed specific and intriguing phenotypes from alteration of spatial memory, reduced sensorimotor coordination, and increased dominance/aggression in mice.
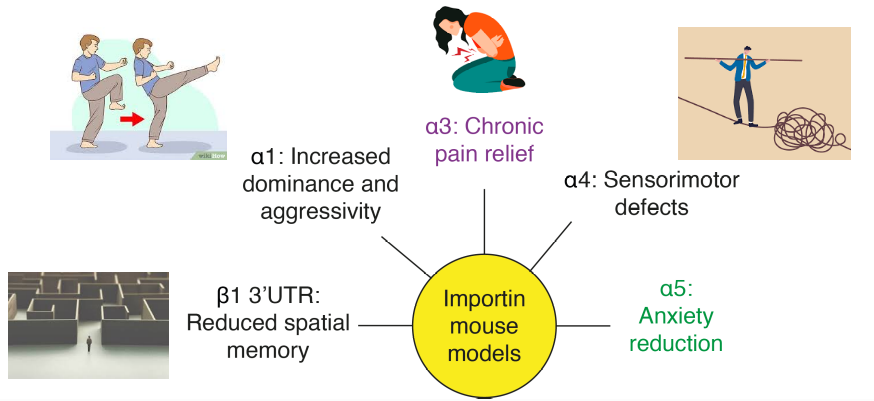
Aside from launching experiments aimed at the dissection of the precise anatomical locus of the importin α5-anxiolysis, our team will perform in-depth analyses of the different models using advanced imaging and RNA-seq, in cell and animal models. This research is expected to generate exciting insights on importin control of transcription and neurobehavioral mechanisms.
Goals: Define the specific contributions of Importin-dependent subcellular transport in neuronal functions and behaviors.
3 – Drug screening approaches
a. In silico drug screening
As demonstrated in our recent works (Marvaldi et al. , 2020; Panayotis et al., 2021), the proposed research will also make the most of RNA-seq and in silico approaches to screen drugs that can present new therapeutics properties (e.g., anxiolytics, painkillers, etc.)
b. Preclinical characterization of newly synthetized molecules.
To date, Rett syndrome remains an incurable pathology. For a long time, the only available treatments aimed at alleviating some symptoms in order to improve the patients’ quality of life. Several pharmacological interventions have been proposed, thanks to the discovery of targets of interest in RTT mouse models. We are collaborating with experts in Medicinal Chemistry and Pharmacology in order to synthetize, characterize, and validate the therapeutic potential of new candidate drugs.
Goals: Identify and validate new pharmacological options to ameliorate anxiety, pain, and comorbidities in neurodevelopmental disorders.
The translational aspect of our research will allow us to make the most of molecular neurobiology and neurogenetics approaches to discover new therapeutic avenues and ameliorate devastating human disorders.
People
Team leader
- Nicolas Panayotis, Research Scientist, PI, CNRS, Team 7 – Molecular Biology of Neuronal Transport, +33 1 76 53 43 96, room 626
Members
- Yoann Leblay, Master Student, Team 7 – Molecular Biology of Neuronal Transport, +33 1 76 53 43 96, room 626
- Prisca Tandabany, Master Student, Team 7 – Molecular Biology of Neuronal Transport, +33 1 76 53 43 96, room 626
Former members
 Marie Manguine, Master Student until 2024, Team 7 – Molecular Biology of Neuronal Transport, +33 1 70 64 99 19
Marie Manguine, Master Student until 2024, Team 7 – Molecular Biology of Neuronal Transport, +33 1 70 64 99 19

Collaborations
- Martin Oheim (Saints-Pères Paris Institute for the Neurosciences, Paris France)
- Jean-Christophe Roux (Marseille Medical Genetics, Marseille, France)
- Zsolt Lenkei (Institute of Psychiatry & Neurosciences, Paris, France)
- Gaetano Angelici (University of Pisa, Italy)
- Marietta Zille (University of Vienna, Austria)
- Mathieu Morel (École Normale Supérieure, Paris, France)
- Mike Fainzilber (Weizmann Institute of Sciences, Rehovot, Israel)
- Michael R. Kreutz (Leibniz Institute for Neurobiology, Magdeburg, Germany)
- Michael Bader, Franziska Rother (Max-Delbrück Center, Berlin-Buch, Germany)
Funding and Institutional Support
- Association Française du Syndrome de Rett (AFSR)
- Fondation de France
- Région Ile-de-France (DIM BioConvS)
- Zeiss Microscopy
- Institut Neurosciences & Cognition (INC)
Recent Publications
Articles
- PTBP1 regulates injury responses and sensory pathways in adult peripheral neurons.
Alber S, Di Matteo P, Zdradzinski MD, Dalla Costa I, Medzihradszky KF, Kawaguchi R, Di Pizio A, Freund P, Panayotis N, Marvaldi L, Doron-Mandel E, Okladnikov N, Rishal I, Nevo R, Coppola G, Lee SJ, Sahoo PK, Burlingame AL, Twiss JL, Fainzilber M : Sci Adv, 2023 - State-of-the-art therapies for Rett syndrome.
Panayotis N, Ehinger Y, Felix MS, Roux JC : Dev Med Child Neurol, 2022 - β-sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice.
Panayotis N, Freund PA, Marvaldi L, Shalit T, Brandis A, Mehlman T, Tsoory MM, Fainzilber M : Cell Rep Med, 2021 - Importin α3 regulates chronic pain pathways in peripheral sensory neurons.
Marvaldi L, Panayotis N, Alber S, Dagan SY, Okladnikov N, Koppel I, Di Pizio A, Song DA, Tzur Y, Terenzio M, Rishal I, Gordon D, Rother F, Hartmann E, Bader M, Fainzilber M : Science, 2020 - Huntingtin phosphorylation governs BDNF homeostasis and improves the phenotype of Mecp2 knockout mice.
Ehinger Y, Bruyère J, Panayotis N, Abada YS, Borloz E, Matagne V, Scaramuzzino C, Vitet H, Delatour B, Saidi L, Villard L, Saudou F, Roux JC : EMBO Mol Med, 2020